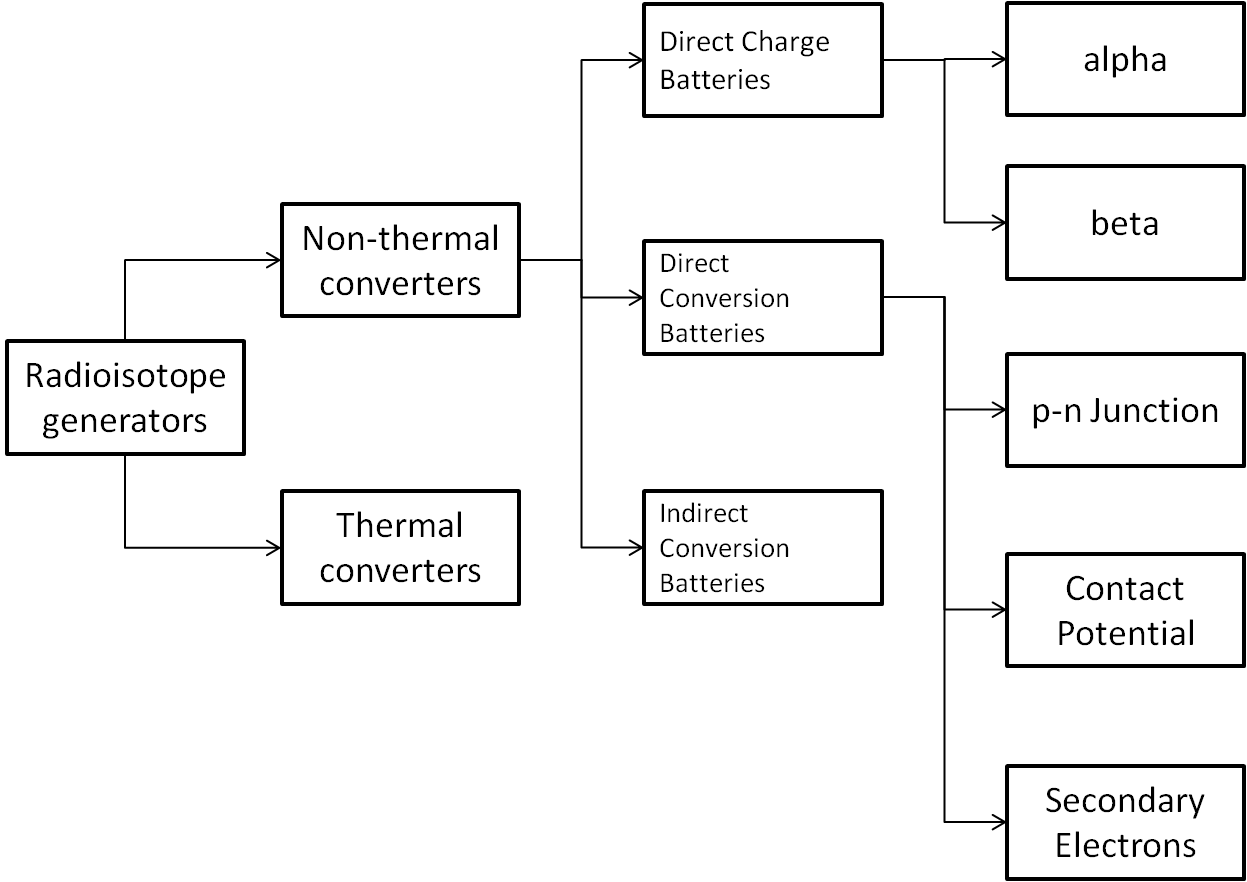
my idea slash question would be:
about how much for enough stronium-90 to approximate the output of the average usa home socket ?
OR
eventually i should say, HOW MUCH would the most cost effective/safe-ER method be of getting a nuclear power source Close to a wall socket??
http://www.politics.ie/forum/education-science/247375-nuclear-powered-cars.html
we dont want to "power an electric car"
but if we had a power source like this built on,
we danmed well could trickle charge the car over 12 hours or something ?
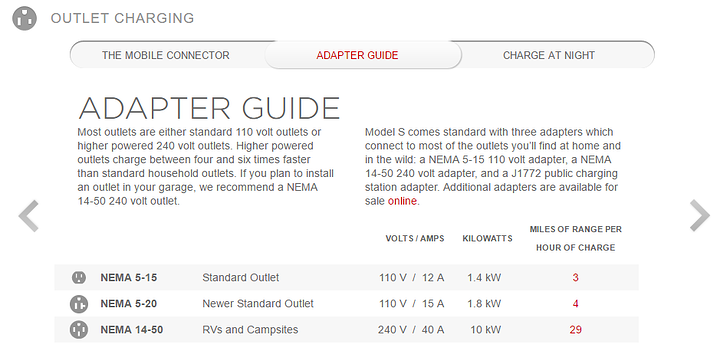
lets take a peek at a tesla car and its claimed charging
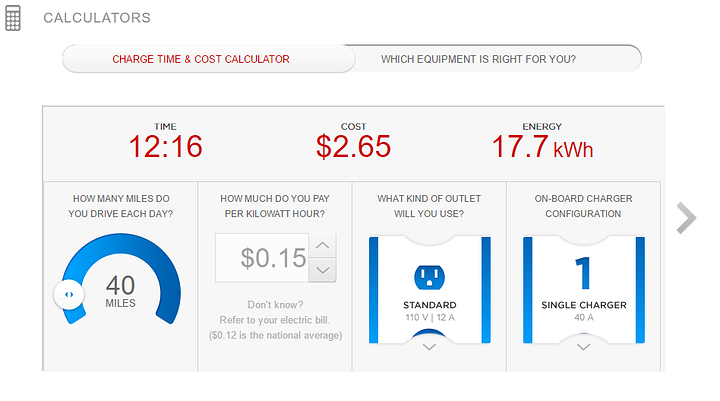
Most modern residential circuits in the usa are 15 or 20 amps,
so we're looking at a max load of either (15A x 120V =) 1800 watts
or
(20A x 120V =) 2400 watts before the breaker trips.
sooo we can say hmm, like 55 miles per 12 hours '??"
@wendell hey man, is your math any good ?
@Eden same question
wanna fart around and theory craft expensive shit ??
running off of random peoples numbers ive seen in a forum,
sourced ,.,..,.neaahhh
according to theese randos posts here in comments section with zero fact checking... (lol rite)
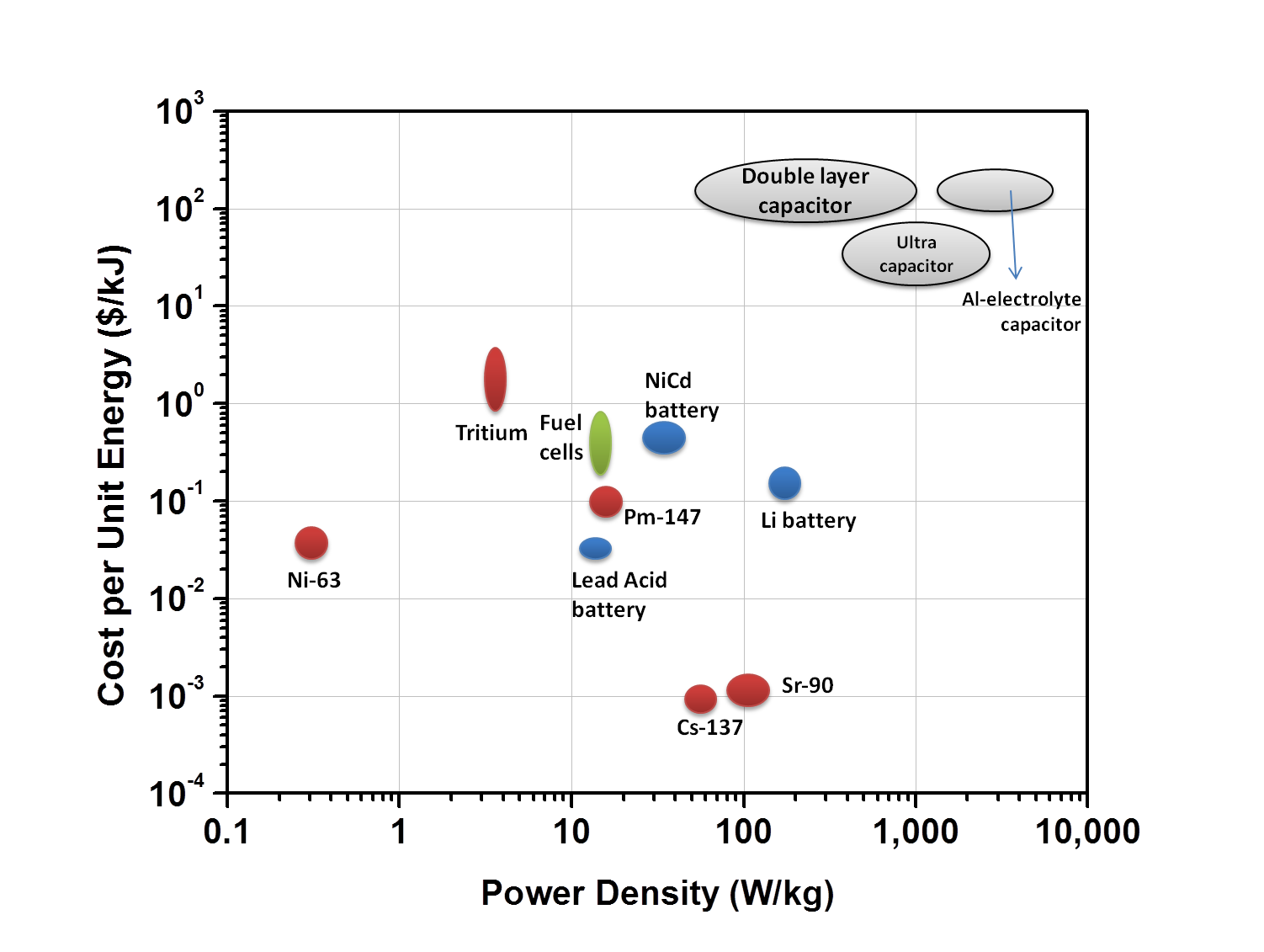
Thad be roughly 6.5kg of Strontium with a 100% efficient converter,
the guy im reading off of says "there was a converter developed in Missouri "recently""
@ 60% efficient,
granted his post was "2 years ago"
so that mass would get bumped up
9.75KG of material ???= 2500Watt output continuous ????
its power density is only 0.46 kilowatts per kilogram.
he claims
" we need around 26 kg of Strontium with a 100%
efficient converter to get 10,000 WATTS"
" but the one developed in Missouri recently is only 60% efficient,
so that gets bumped up to 43.3kg, which is just under 100 lbs of Strontium"
Strontium-90 also requires little shielding, as it decays
by β emission, with negligible γ emission. While its half life of 28.8 years
someone good with this type of shit plz fact check my stupid ass.
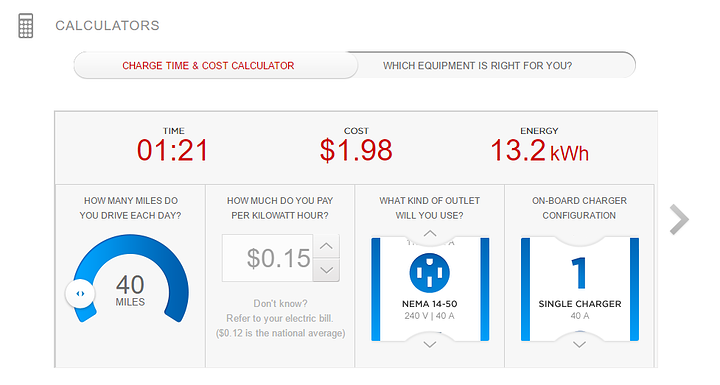
if we go up to 240 v and 40amp
we REALLY cut down the charge time thoooo
which isnt the point
the whole idea here was how much would the LOWEST ammount BE
that would let us in THEORY
trickle charge a car overnite ?
so the all important question,
how much is different materials that could fit the bill ?
stronium-90 is the substance this guy was talkin bout
Molecular Weight: 89.908 g/mol
Cost, pure: $100 per 100g
Cost, bulk: $ per 100g
but wat about our isotope ?
Strontium-85 can be produced by irradiation of rubidium-85 with accelerated protons or deuterons.
Multi-Agency Radiological Laboratory Analytical Protocols Manual Volume II: Chapters 10-17 and Appendix F. (July 2004) p 14-155 NUREG-1576, EPA 402-B-04-001B, NTIS PB2004-105421. Available from, as of October 12, 2006: http://www.nrc.gov/reading-rm/doc-collections/nuregs/staff/sr1576/sr1576v2.pdf
Strontium Sr 38
pure $1000
Strontium Element Facts
Uses of Strontium
Strontium is used for producing glass (cathode ray tubes) for color televisions. It is also used in producing ferrite ceramic magnets and in refining zinc.
The world’s most accurate atomic clock, accurate to one second in 200 million years, has been developed using strontium atoms.
Strontium salts are used in flares and fireworks for a crimson color.
Strontium chloride is used in toothpaste for sensitive teeth.
Strontium oxide is used to improve the quality of pottery glazes.
The isotope 90Sr is one of the best long-lived, high-energy beta emitters known. It is used in cancer therapy.
Abundance and Isotopes
Abundance earth’s crust: 370 parts per million by weight, 87 parts per million by moles
Abundance solar system: 50 parts per billion by weight, 0.7 parts per billion by moles
Cost, pure: $100 per 100g
Cost, bulk: $ per 100g
Source: Strontium is never found free in nature. The principal strontium ores are celestine (strontium sulfate, SrSO4) and strontianite (strontium carbonate, SrCO3). The main commercial process for strontium metal production is reduction of strontium oxide with aluminum.
Isotopes: Strontium has 28 isotopes whose half-lives are known, with mass numbers 75 to 102. Naturally occurring strontium is a mixture of its four stable isotopes and they are found in the percentages shown: 84Sr (0.6%), 86Sr (9.9%), 87Sr (7.0%) and 88Sr (82.6%).
References
1. Mary Elvira Weeks, Discovery of the Elements., Journal of Chemical Education (June 1932) p1046.
2. T. K. Kenyon, Science and Celebrity: Humphry Davy’s Rising Star., Chemical Heritage Magazine, (2008/9 edition).
Cite this Page
For online linking, please copy and paste one of the following: