I will be including wikipedia links to common terms to serve as a sort of glossary 
This post is as complete as I can make it for now and is always a work in progress. It may turn into a wiki about Ethanol
DISCLAIMER
Please do NOT run on higher levels of ethanol in a non FFV above 25 percent. Please tune a vehicle properly if considering use. I am not responsible for damage if you take my route
A little background
So your burning dinoline aka gasoline in your cars. You hear a lot of stuff about emissions and improving the environment. Lets talk about that for a bit.
Gasoline
Gasoline as you all know , is a colorless to straw yellow liquid we burn in our vehicles. This liquid is petroleum-derived flammable liquid. This fuel is primarily used in spark-ignited internal combustion engines. The yield of gasoline to a barrel of crude oil is roughly 70 liters for every 162 liters of crude oil or roughly 43 percent from its source. REMEMBER 43 percent we will be using this number later!
The main components we find in modern gasoline are: isooctane, butane, 3-ethyltoluene, and the octane enhancer MTBE. This gasoline if properly refined will have a smaller ratio of isoheptane aka C7 chains and similar functional groups. These are responsible for lowering the octane number while higher levels of isooctane are responsible for a higher octane number. We use these numbers to create an Anti Knock Index using the method of RON + MON /2 while europe typically differs using RON or research octane number as the primary number rating vs averaging it with the MON or Motor Octane Number.
Left Top: ISOOCTANE
Left Bottom: BUTANE
Center: 4-Ethyltoluene
Right: Methyl tertiary-butyl ether (MTBE)
As you can see gasoline is a mixture of organic molecules. This presents an issue when burning the fluid. Its combustion is volatile but also unpredictable and makes it a rather dirty none homogeneous fuel. Certain pockets will burn at different rates and different amounts of heat. Pretty cool huh? So how do we burn it reliably. Well we refine it into mostly C4-C12 chains preferably C8 or above. These hydrogen bonds onto this carbon and oxygen based molecule AKA a HYDROCARBON is able to release a lot of energy giving gasoline another value you should remember for this post. This property is an energy density of gasoline at a glorious 114,405 BTU/US gal (BTU stands for her majesties…-- egh cough right British thermal units). Gasoline is also toxic and cancer causing.
Combustion of gasoline is a chemical reaction as you probably know. Lets step you through it just a bit. The stoichiometric combustion of gasoline with pure oxygen is as follows if using a stoichiometric ration of 14.65:1. We are going to step through 4 equations showing why this is not always the case.
C8H18 + 12.5 O2 → 8 CO2 + 9 H2O (EQ 1)
Great there is the ideal and educational case. Guess what thats fantasy haha  man oh man if it only worked that way. So lets get slightly more realistic. If you burn gasoline with air instead of oxygen (assuming 21% oxygen 79% nitrogen):
man oh man if it only worked that way. So lets get slightly more realistic. If you burn gasoline with air instead of oxygen (assuming 21% oxygen 79% nitrogen):
C8H18 + 12.5 (O2 + 3.76 N2) → 8 CO2 + 9H2O + 47 N2 (EQ 2)
The second equation is simply the first one with nitrogen or N2 added. In ideal case the oxygen in the air reacts with gasoline then forms carbon-dioxide and water vapor. Well this isnt the case and varies based on the temperature of the combustion chamber both post and pre combustion as well during and throughout it. Not to mention we have another issue that I mentioned previously. The combustion is not stoichiometric everywhere but rather we have fuel lean and fuel rich zones. In such cases (still ideal conditions) we have oxygen or carbon-monoxide in the products. This brings us to our condition varying ideal equations without NOx which is produced which will occur if the flame temperature reaches 2800 F. This can happen believe it or not  but anyways here we go:
but anyways here we go:
C8H18 + 12.5α (O2 + 3.76 N2) → 8CO2 + 9H2O + 47N2 + (α-1) O2 (EQ 3)*
When my dummy variable alpha is greater than 1 this means that we have excess oxidizer and the combustion is fuel lean. Note that for α =1 the third equation is same as the second equation.
C8H18 + a (O2+3.76 N2) → b CO2 + c CO + d H2O + (3.76 a) N2 (EQ 4)
The dummy variables a, b, c and d are some constants which can be found from the chemical equilibrium ( EQ 1) and element balance ( EQ 3) and really get to complicated for this post. This is the fuel rich case and we have unburned hydrocarbon/fuel. See I bet your feeling confused already well guess what!? Real life burning of gasoline still isnt this simple.
However in real life these equations are changed a little bit from here and considered a fairly good enough model. If you examine the exhaust port of the combustion chamber of an internal combustion engine, you will have CO, CO2, H2O, NOx, SOx, x is a number like 1 or 2 depending on number of Oxidation bonds. Engineers working on automobiles in the industry tend to lower NOx and SOx emissions under a threshold or try to design as such because they want to be green and yes the earth matters.
Thanks for bearing with the organic chemistry lesson. We now know gasoline is a fairly dirty fuel, its toxic, its not easy to model, and it can be tricky to raise the octane of for efficient combustion. However we do also know that it has superior energy density 
Let’s talk about Ethanol
Ethanol is part of the function group of Ethyl Alcohols. Its often mixed into gasoline above to raise the octane and improve the combustion characteristics often cleaning up the combustion of the fuel. Before anybody rants and says this isnt always true I have found a decent video demonstration:
Now that youve seen the physical proof lets move forward with ethanol. Fun fact its also part of your wonder alcoholic beverages and making it is nearly identical to how you make moonshine. Though you would not want to drink straight ethanol (E95) as it could kill you via intoxication. E95 is referred to as hydrous ethanol. It is the highest content of alcohol you can refine in a fractional distiller. Often we are told water is bad and ethanol is corrosive we will get into the truth behind this a bit later on.
Chemical Make Up and Combustion
The chemical diagram of the base group of Ethyl Alcohol is as follows. Its a two carbon atom alcohol. Note that you can tell its an alcohol because of the top right O-H bond. Alcohols are part of the OH functional group  . Here enjoy this beautiful graphic. Note the skeletal digramn. For the curious the solid wedges represent bonds that point out of the plane of the screen or towards you the observer in a 3D sense and of course the hashed wedges mean the opposite direction. We are not going to get into this. Start here if you want to learn
. Here enjoy this beautiful graphic. Note the skeletal digramn. For the curious the solid wedges represent bonds that point out of the plane of the screen or towards you the observer in a 3D sense and of course the hashed wedges mean the opposite direction. We are not going to get into this. Start here if you want to learn 
SO an alcohol is a hydrocarbon. Its flammable. Its relatively easy to store. Hey we can use it to burn as a fuel right and it will be just like gasoline LETS DO EEET! LOL turn down those RPMs for a moment its not that simple. Ethanol contains roughly 78,150 BTU / gal and in practice this can very slightly. Let us compare this to gasoline by creating a Gasoline Gallon Equivalency. This fairly simple. Take 78150/114450 and we get roughly 0.6828 so pure E95 hydrous ethanol contains 68.28 % of the energy content of gasoline. This means that we need roughly 31.72 % more gasoline to create the same amount of work all things being equal. This means at MAX you need 1.3172 the amount of fuel into the chamber in terms of consuption to run your vehicle. For all intents and purposes Ill skip the combustion formula for now as unburned alcohols can oxidize into aldehydes in tiny amounts and I dont want to get into full alcohol combustion. However an important number to realize is that while ethanol has an ideal stoich of 9.6:1 ratio wise it can be efficiently burned at up to 12:1 without engine damage which will be close to the ratio that a non Flex fuel vehicle will burn it at.
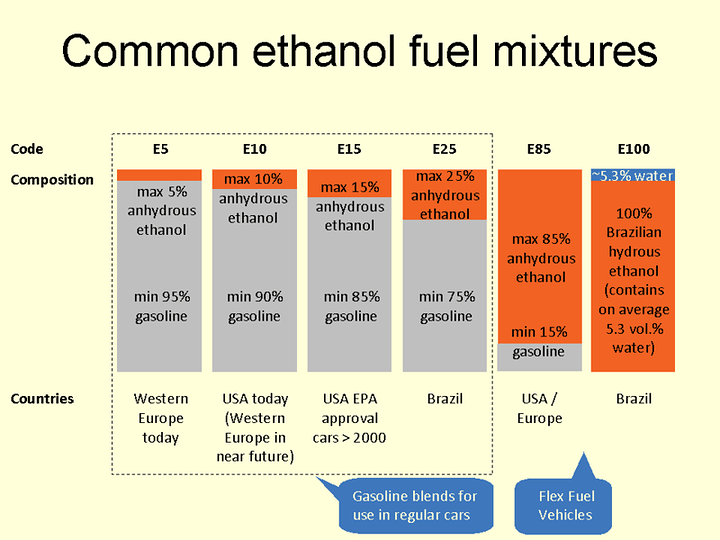
Its often mixed with gasoline. Heres a diagramn
Dispelling the myths!
MPG
Alright lets get rid of this boomer age statement: “Oh my god ethanol trashed my fuel economy and not I get 15 mpg bla bla more running E0 fuel”
Guess what its 100 % a meme. Its a complete logical fallacy. How can you get that much more MPG? You cant period said done no buts and ifs or any of that. Why? Glad you asked. E10 contains up to 10 percent ethanol. This is your standard pump gas and sometimes you may see E15. Realistically they have 5 and 10 percent on average respectively. So 10 percent ethanol. Great well then lets do some simple math to calculate our BTUs which will directly translate to the percentage of MPG changes
BTU (E10) (Worst Case): (78150(0.1)+114450(.9))/(114450) = 0.96 or 96 percent that of PURE gasoline. Great so we lost 4 percent fuel economy. Lets translate that to MPG. Lets say this average boomer’s car gets about 26 MPG. Lets calculate the maximum MPG gain. This gain is equal to 26*1.04 which is 27.04 MPG. Wow a whole MPG! Woot congrats you gained some fuel economy and thats all you could gain PERIOD. Any other gains are in your head but losses are also possible. The addition of ethanol acts as an oxygenate which can improve the amount of work output of your enegine if properly mixed. Unfortunately E10 is not ideal and results in lean pockets in your engine which can reduce fuel economy and this is the main issue people see. There is a saving grace. An ideal mixture of gasoline to ethanol is actually E33 and this is due to the non linear nature of mixtures. It turns out E33 while it should have have roughly 11 percent less fuel economy. In practice and in my own practice as well I find this number to be closer to 8. There are many theories as to why. Primary reasons for the discrepancy are due to the oxygenation and excess oxygen provided to gasoline as well as the higher octane of ethanol. This allows for your engines ECU (Engine Control Unit) to advance the timing of the engine and fully utilize the fuel thermodynamically.
It blew so and so’s engine up corn fuel bad
LOL another misinformed statement. Ethanol cannot be the cause of your engine detonating because it has a far superior AKI or anti knock index. Here is a chart that has a huge list of various fuels for example. Scrolling to E85 or anything with ethanol will show you that the AKI is superior
Time Saver:
Pure E95 Ethanol: 108.6
E85: 104.7
E33: 93.1
E15: 90.1
E10: 89
E5: 88
E0: 87
Pure Gasoline without MTBE: 83 or 85 (sold in some rocky mountain states DO NOT RUN THIS IN A MODERN CAR)
So an engine cannot detonate or knock to death when the octane is higher period said done. It is probably the result of poor maintenance or some off the shelf crap snake oil chemical. Bottom line is ethanol wont harm engines designed since the E10 transition in the 90’s which includes damaging fuel lines. It cannot and will not damage rubber fuel lines since circa 1992 when the switched over to polar chemical resistant lines. Ethanol is a polar chemical. Any line tank and system rated for E10 can handle E85 or higher. There are far too many lies and fake science surrounding this eating of fuel lines. The video I posted should show this as well. Lets show case this. Heres a video from the same guy using small engine kits which is the source of almost all the modern day complaints proving its a myth and your being lied to. Yeah yeah sample size of 1 isnt a good measure. Guess what the scientific method is. I recreated the same experiment and so can you to verify his results.
Water Absorption
Ethanol is hydroscopic this is true. However the rate at which it can do this is VASTLY overstated. Lets show one more video showing a 33 percent ethanol start up after sitting for a year in what is not the most sealed tank:
Try that with regular non stabilized gasoline! LOL youll be tearing the machine apart just like I had to with a lawn mower. But hey dont take my word for it you can always run an experiment like he did. I have done a similar experiment with lawn mowers yielding similar results. Well its not all fine and dandy SOMETIMES water can get in higher concentrations not due to ethanol and the ethanol will emulsify the water temporarily but something called phase separation will occur. Ethanol in its pure form can contain up to 5 percent water hence E95 and not phase separate. In this form ethanol will also NOT draw anymore water because the ethanol has satisfied its azeotropic equilibrium. However this presents a problem ethanol cannot satisfy this equilibrium if cosolvents are used in conjunction with higher volumes of gasoline aka 80+% or E20 - E0. This issue is lessened the higher the mix of ethanol E33 experiences less and if the mixture of ethanol is greater than 71 percent phase separation will never occur and the water content will never be in excess of 3.5 percent. Water is not always bad in an engine and its always present in some form some where. In fact water can lower the reactivity of oxidative properties of ethanol making it more stable and less corrosive. The Netherlands found this out by using hydrous ethanol. You can read on this here:
The United states but not brazil uses anahydrous ethanol as of 2018. Anahydrous is expensive and also makes ethanol very hydroscopic and corrosive due to needing those extra water molecules for stabilization. Its estimated that ethanol fuels could be 30-48 percent cheaper if we chose not to remove that last bit of water. Also due to the lack of phase seperation above 70 percent ethanol as discussed earlier it can also be used in winter without worry of freezing down to -114.1°C for pure ethanol and -98 C for E70. E70 does not have cold start issues so long as the ethanol content does not exceed 75 percent. Anywhere above that and you have cold start issues. This is why E85 has a lower content of ethanol in the winter 
My engine runs smoother on E10
This is only partially somewhat true in certain circumstances. Let’s overview all the conditions where smoother operation is more likely:
E0 all cars can run pure gasoline. This will yield smooth consistent operation. E33 The ideal mixture tends to run exceptionally smooth with very little RPM fluctuation at idle. Mixtures above E51 tend can be hit or miss depending on some conditions but generally run smoother. E95 like the case of E0 being a pure liquid of the fuel will run very smooth in fact smoother than E0 with a good tune (most cars need a tune for this)… This is due to it being a pure homogeneous mixture of only one time of flammable liquid. The air fuel mix is extremely consistent
Whats stopping us from running it?
Honestly, just an ECU tune 99 percent of the time. If you have a non flexible fuel you can have the fuel mapped to be adaptive or you can have multiple tunes one for gasoline or gasohol (E10-15) and one for E70 which is the perfect content to tune to for running E85 (contains 55-83 percent ethanol). Due to being smack in the middle the ECU can compensate for the slight variances without issue. You can run it in a normal non ffv car. However a lot of the time you will encounter a check engine light beyond 35 percent however below that amount you will suffer no I’ll effects. Engine temperatures will be normal if not slightly cooler in a direct injection engine and on a port injection engine they will be normal or slight above due to the differences of those two combustion methods. Either way so long as you don’t have enough to set off your check engine light and you’ve blended an amount your vehicle can compensate for you will be just fine. The check engine light isnt bad. It just tells you that you are running leaner than the rigid mapping from the factory can handle. Lean can be bad in a turbo charged engine. DO NOT DO THIS IN A TURBO ENGINE WITHOUT A TUNE. If you want to do this in a turbo engine without a tune do not blend more than 23 percent ethanol or you risk actually running to lean in boosted conditions which is bad. I hope that all caps gets your attention.
Personal Use and Testing
I do have some numbers for yall 
Things to note:
Highway driving determined by speed:
<=45 is city
46 is highway
Ethanol Testing kit used
BASELINE
Details below about driving and how I monitored this is just a baseline section. Upon thinking about ethanol my car had a ton of miles on it. I started recording with the app torque detailed below at roughly 73k miles on this 2009 Ford Focus that wasnt E85 tuned until later  SO here we have a regular non FFV car doing just fine. Upon reaching 200 tanks measured with the torque app I decided to make the switch over to various amounts of ethanol and I decided to carry out this somewhat scientific test for 200 tank recordings
SO here we have a regular non FFV car doing just fine. Upon reaching 200 tanks measured with the torque app I decided to make the switch over to various amounts of ethanol and I decided to carry out this somewhat scientific test for 200 tank recordings  I figured that would be only fair. Ive always been particularly meticulous about running the tank down to the under my quarter tank and then filling up. I rounded some numbers up to try to keep things easier to do the math and the differences werent that great
I figured that would be only fair. Ive always been particularly meticulous about running the tank down to the under my quarter tank and then filling up. I rounded some numbers up to try to keep things easier to do the math and the differences werent that great  If you want the unrounded version I can always post it but I dont really want to because it doesnt matter too much.
If you want the unrounded version I can always post it but I dont really want to because it doesnt matter too much.
Table 1
Baseline Table
| Tank # | E Content | Gallons Used (rounded up) | Distance Traveled | MPG | Highway % | City % | AVG | LTFT (AVG %) |
|---|---|---|---|---|---|---|---|---|
| 1 | – | 8 | 329.67 | 36.63 | 84 | 16 | 1 | |
| 2 | – | 10 | 349.2 | 34.92 | 87 | 13 | 2 | |
| 3 | – | 11 | 405.46 | 36.86 | 70 | 30 | 3 | |
| 4 | – | 12 | 421.8 | 35.15 | 83 | 17 | 1 | |
| 5 | – | 11 | 376.2 | 34.2 | 82 | 18 | 3 | |
| 6 | – | 12 | 437.76 | 36.48 | 70 | 30 | 3 | |
| 7 | – | 8 | 276.48 | 34.56 | 81 | 19 | 2 | |
| 8 | – | 12 | 427.68 | 35.64 | 83 | 17 | 3 | |
| 9 | – | 12 | 433.2 | 36.1 | 84 | 16 | 1 | |
| 10 | – | 8 | 274.4 | 34.3 | 85 | 15 | 1 | |
| 11 | – | 8 | 285.12 | 35.64 | 85 | 15 | 1 | |
| 12 | – | 9 | 338.58 | 37.62 | 83 | 17 | 1 | |
| 13 | – | 10 | 362.6 | 36.26 | 87 | 13 | 3 | |
| 14 | – | 12 | 421.8 | 35.15 | 81 | 19 | 2 | |
| 15 | – | 9 | 316.35 | 35.15 | 75 | 25 | 3 | |
| 16 | – | 13 | 476.19 | 36.63 | 72 | 28 | 1 | |
| 17 | – | 10 | 339.5 | 33.95 | 82 | 18 | 3 | |
| 18 | – | 8 | 263.84 | 32.98 | 79 | 21 | 3 | |
| 19 | – | 13 | 428.74 | 32.98 | 80 | 20 | 3 | |
| 20 | – | 8 | 279.36 | 34.92 | 82 | 18 | 3 | |
| 21 | – | 8 | 263.84 | 32.98 | 69 | 31 | 2 | |
| 22 | – | 8 | 273.6 | 34.2 | 80 | 20 | 2 | |
| 23 | – | 12 | 415.8 | 34.65 | 87 | 13 | 2 | |
| 24 | – | 11 | 369.6 | 33.6 | 81 | 19 | 3 | |
| 25 | – | 11 | 413.82 | 37.62 | 87 | 13 | 1 | |
| 26 | – | 13 | 474.24 | 36.48 | 72 | 28 | 1 | |
| 27 | – | 11 | 413.82 | 37.62 | 78 | 22 | 3 | |
| 28 | – | 11 | 413.82 | 37.62 | 73 | 27 | 1 | |
| 29 | – | 8 | 276.48 | 34.56 | 78 | 22 | 1 | |
| 30 | – | 9 | 335.16 | 37.24 | 79 | 21 | 3 | |
| 31 | – | 11 | 359.04 | 32.64 | 75 | 25 | 1 | |
| 32 | – | 11 | 401.28 | 36.48 | 77 | 23 | 2 | |
| 33 | – | 9 | 317.52 | 35.28 | 69 | 31 | 3 | |
| 34 | – | 10 | 351.5 | 35.15 | 88 | 12 | 2 | |
| 35 | – | 9 | 299.25 | 33.25 | 77 | 23 | 3 | |
| 36 | – | 10 | 368.6 | 36.86 | 87 | 13 | 1 | |
| 37 | – | 9 | 308.7 | 34.3 | 75 | 25 | 3 | |
| 38 | – | 13 | 432.25 | 33.25 | 77 | 23 | 1 | |
| 39 | – | 12 | 410.4 | 34.2 | 79 | 21 | 3 | |
| 40 | – | 11 | 390.72 | 35.52 | 85 | 15 | 2 | |
| 41 | – | 12 | 439.56 | 36.63 | 80 | 20 | 2 | |
| 42 | – | 12 | 426.24 | 35.52 | 77 | 23 | 3 | |
| 43 | – | 8 | 293.04 | 36.63 | 80 | 20 | 1 | |
| 44 | – | 8 | 263.84 | 32.98 | 83 | 17 | 2 | |
| 45 | – | 12 | 414.72 | 34.56 | 71 | 29 | 3 | |
| 46 | – | 9 | 331.74 | 36.86 | 73 | 27 | 1 | |
| 47 | – | 8 | 277.2 | 34.65 | 88 | 12 | 2 | |
| 48 | – | 12 | 403.92 | 33.66 | 77 | 23 | 1 | |
| 49 | – | 8 | 279.36 | 34.92 | 82 | 18 | 1 | |
| 50 | – | 13 | 489.06 | 37.62 | 73 | 27 | 3 | |
| 51 | – | 9 | 290.7 | 32.3 | 70 | 30 | 2 | |
| 52 | – | 13 | 419.9 | 32.3 | 87 | 13 | 3 | |
| 53 | – | 8 | 285.12 | 35.64 | 71 | 29 | 1 | |
| 54 | – | 12 | 414.72 | 34.56 | 86 | 14 | 3 | |
| 55 | – | 8 | 284.16 | 35.52 | 72 | 28 | 1 | |
| 56 | – | 11 | 401.28 | 36.48 | 73 | 27 | 3 | |
| 57 | – | 10 | 356.4 | 35.64 | 76 | 24 | 1 | |
| 58 | – | 13 | 444.6 | 34.2 | 71 | 29 | 2 | |
| 59 | – | 11 | 392.04 | 35.64 | 79 | 21 | 1 | |
| 60 | – | 9 | 308.7 | 34.3 | 72 | 28 | 2 | |
| 61 | – | 13 | 453.96 | 34.92 | 70 | 30 | 1 | |
| 62 | – | 11 | 388.08 | 35.28 | 74 | 26 | 1 | |
| 63 | – | 10 | 329.8 | 32.98 | 75 | 25 | 3 | |
| 64 | – | 9 | 328.32 | 36.48 | 77 | 23 | 2 | |
| 65 | – | 12 | 414.72 | 34.56 | 72 | 28 | 2 | |
| 66 | – | 10 | 362.6 | 36.26 | 76 | 24 | 1 | |
| 67 | – | 10 | 342 | 34.2 | 73 | 27 | 1 | |
| 68 | – | 13 | 445.9 | 34.3 | 70 | 30 | 1 | |
| 69 | – | 8 | 277.2 | 34.65 | 71 | 29 | 2 | |
| 70 | – | 8 | 274.4 | 34.3 | 76 | 24 | 1 | |
| 71 | – | 8 | 282.24 | 35.28 | 81 | 19 | 1 | |
| 72 | – | 9 | 319.68 | 35.52 | 88 | 12 | 3 | |
| 73 | – | 11 | 380.16 | 34.56 | 85 | 15 | 3 | |
| 74 | – | 9 | 290.7 | 32.3 | 82 | 18 | 2 | |
| 75 | – | 10 | 351.5 | 35.15 | 73 | 27 | 3 | |
| 76 | – | 9 | 316.35 | 35.15 | 86 | 14 | 1 | |
| 77 | – | 13 | 445.9 | 34.3 | 73 | 27 | 1 | |
| 78 | – | 10 | 376.2 | 37.62 | 69 | 31 | 1 | |
| 79 | – | 9 | 338.58 | 37.62 | 84 | 16 | 2 | |
| 80 | – | 9 | 323.01 | 35.89 | 74 | 26 | 3 | |
| 81 | – | 8 | 276.48 | 34.56 | 77 | 23 | 3 | |
| 82 | – | 12 | 399 | 33.25 | 84 | 16 | 1 | |
| 83 | – | 9 | 335.16 | 37.24 | 77 | 23 | 2 | |
| 84 | – | 9 | 299.88 | 33.32 | 82 | 18 | 2 | |
| 85 | – | 11 | 370.26 | 33.66 | 70 | 30 | 3 | |
| 86 | – | 10 | 343 | 34.3 | 87 | 13 | 1 | |
| 87 | – | 12 | 437.76 | 36.48 | 87 | 13 | 3 | |
| 88 | – | 9 | 319.68 | 35.52 | 87 | 13 | 1 | |
| 89 | – | 13 | 433.16 | 33.32 | 78 | 22 | 3 | |
| 90 | – | 11 | 376.2 | 34.2 | 87 | 13 | 2 | |
| 91 | – | 8 | 266 | 33.25 | 69 | 31 | 1 | |
| 92 | – | 12 | 399 | 33.25 | 88 | 12 | 1 | |
| 93 | – | 11 | 359.04 | 32.64 | 86 | 14 | 3 | |
| 94 | – | 8 | 288.8 | 36.1 | 80 | 20 | 2 | |
| 95 | – | 10 | 352.8 | 35.28 | 82 | 18 | 1 | |
| 96 | – | 11 | 384.12 | 34.92 | 71 | 29 | 1 | |
| 97 | – | 10 | 342 | 34.2 | 74 | 26 | 1 | |
| 98 | – | 8 | 284.16 | 35.52 | 80 | 20 | 3 | |
| 99 | – | 12 | 430.68 | 35.89 | 73 | 27 | 3 | |
| 100 | – | 10 | 345.6 | 34.56 | 84 | 16 | 3 | |
| 101 | – | 12 | 403.92 | 33.66 | 83 | 17 | 3 | |
| 102 | – | 11 | 381.15 | 34.65 | 81 | 19 | 2 | |
| 103 | – | 13 | 445.9 | 34.3 | 73 | 27 | 3 | |
| 104 | – | 12 | 442.32 | 36.86 | 72 | 28 | 2 | |
| 105 | – | 9 | 311.04 | 34.56 | 88 | 12 | 2 | |
| 106 | – | 9 | 323.01 | 35.89 | 81 | 19 | 1 | |
| 107 | – | 10 | 336 | 33.6 | 72 | 28 | 2 | |
| 108 | – | 9 | 302.4 | 33.6 | 81 | 19 | 1 | |
| 109 | – | 10 | 356.4 | 35.64 | 75 | 25 | 2 | |
| 110 | – | 13 | 479.18 | 36.86 | 80 | 20 | 1 | |
| 111 | – | 8 | 258.4 | 32.3 | 76 | 24 | 3 | |
| 112 | – | 11 | 362.78 | 32.98 | 79 | 21 | 2 | |
| 113 | – | 9 | 319.68 | 35.52 | 78 | 22 | 1 | |
| 114 | – | 13 | 474.24 | 36.48 | 88 | 12 | 3 | |
| 115 | – | 9 | 305.55 | 33.95 | 88 | 12 | 3 | |
| 116 | – | 8 | 300.96 | 37.62 | 70 | 30 | 3 | |
| 117 | – | 8 | 266 | 33.25 | 77 | 23 | 3 | |
| 118 | – | 9 | 299.88 | 33.32 | 82 | 18 | 3 | |
| 119 | – | 9 | 329.67 | 36.63 | 79 | 21 | 1 | |
| 120 | – | 8 | 281.2 | 35.15 | 74 | 26 | 1 | |
| 121 | – | 11 | 388.08 | 35.28 | 81 | 19 | 1 | |
| 122 | – | 13 | 432.25 | 33.25 | 77 | 23 | 1 | |
| 123 | – | 12 | 427.68 | 35.64 | 83 | 17 | 3 | |
| 124 | – | 13 | 441.35 | 33.95 | 75 | 25 | 2 | |
| 125 | – | 11 | 381.15 | 34.65 | 80 | 20 | 1 | |
| 126 | – | 13 | 424.32 | 32.64 | 69 | 31 | 1 | |
| 127 | – | 8 | 261.12 | 32.64 | 81 | 19 | 3 | |
| 128 | – | 8 | 276.48 | 34.56 | 71 | 29 | 1 | |
| 129 | – | 12 | 430.68 | 35.89 | 78 | 22 | 3 | |
| 130 | – | 8 | 279.36 | 34.92 | 71 | 29 | 3 | |
| 131 | – | 10 | 323 | 32.3 | 69 | 31 | 3 | |
| 132 | – | 11 | 365.75 | 33.25 | 75 | 25 | 1 | |
| 133 | – | 13 | 445.9 | 34.3 | 83 | 17 | 3 | |
| 134 | – | 11 | 392.04 | 35.64 | 69 | 31 | 2 | |
| 135 | – | 13 | 463.32 | 35.64 | 75 | 25 | 3 | |
| 136 | – | 11 | 376.2 | 34.2 | 78 | 22 | 1 | |
| 137 | – | 9 | 323.01 | 35.89 | 72 | 28 | 2 | |
| 138 | – | 11 | 359.04 | 32.64 | 75 | 25 | 3 | |
| 139 | – | 9 | 302.4 | 33.6 | 73 | 27 | 3 | |
| 140 | – | 13 | 484.12 | 37.24 | 71 | 29 | 2 | |
| 141 | – | 13 | 456.95 | 35.15 | 82 | 18 | 2 | |
| 142 | – | 9 | 302.94 | 33.66 | 79 | 21 | 3 | |
| 143 | – | 10 | 329.8 | 32.98 | 72 | 28 | 2 | |
| 144 | – | 10 | 323 | 32.3 | 85 | 15 | 3 | |
| 145 | – | 8 | 284.16 | 35.52 | 83 | 17 | 1 | |
| 146 | – | 9 | 311.85 | 34.65 | 74 | 26 | 3 | |
| 147 | – | 10 | 336.6 | 33.66 | 71 | 29 | 3 | |
| 148 | – | 13 | 424.32 | 32.64 | 73 | 27 | 1 | |
| 149 | – | 10 | 345.6 | 34.56 | 83 | 17 | 1 | |
| 150 | – | 12 | 399.84 | 33.32 | 81 | 19 | 1 | |
| 151 | – | 13 | 479.18 | 36.86 | 85 | 15 | 3 | |
| 152 | – | 9 | 319.68 | 35.52 | 88 | 12 | 1 | |
| 153 | – | 10 | 355.2 | 35.52 | 72 | 28 | 3 | |
| 154 | – | 8 | 266 | 33.25 | 73 | 27 | 3 | |
| 155 | – | 13 | 474.24 | 36.48 | 81 | 19 | 2 | |
| 156 | – | 10 | 333.2 | 33.32 | 78 | 22 | 1 | |
| 157 | – | 9 | 311.85 | 34.65 | 84 | 16 | 2 | |
| 158 | – | 13 | 449.28 | 34.56 | 83 | 17 | 1 | |
| 159 | – | 13 | 461.76 | 35.52 | 78 | 22 | 2 | |
| 160 | – | 8 | 268.8 | 33.6 | 70 | 30 | 2 | |
| 161 | – | 10 | 362.6 | 36.26 | 69 | 31 | 3 | |
| 162 | – | 13 | 444.6 | 34.2 | 81 | 19 | 2 | |
| 163 | – | 8 | 266 | 33.25 | 80 | 20 | 1 | |
| 164 | – | 11 | 384.12 | 34.92 | 72 | 28 | 2 | |
| 165 | – | 10 | 368.6 | 36.86 | 82 | 18 | 1 | |
| 166 | – | 9 | 335.16 | 37.24 | 69 | 31 | 1 | |
| 167 | – | 8 | 277.2 | 34.65 | 85 | 15 | 2 | |
| 168 | – | 8 | 297.92 | 37.24 | 76 | 24 | 3 | |
| 169 | – | 11 | 370.26 | 33.66 | 69 | 31 | 2 | |
| 170 | – | 10 | 326.4 | 32.64 | 73 | 27 | 2 | |
| 171 | – | 10 | 372.4 | 37.24 | 86 | 14 | 2 | |
| 172 | – | 9 | 331.74 | 36.86 | 69 | 31 | 2 | |
| 173 | – | 8 | 290.08 | 36.26 | 81 | 19 | 3 | |
| 174 | – | 8 | 277.2 | 34.65 | 81 | 19 | 3 | |
| 175 | – | 11 | 362.78 | 32.98 | 85 | 15 | 3 | |
| 176 | – | 11 | 369.6 | 33.6 | 84 | 16 | 3 | |
| 177 | – | 10 | 345.6 | 34.56 | 84 | 16 | 3 | |
| 178 | – | 13 | 436.8 | 33.6 | 76 | 24 | 3 | |
| 179 | – | 9 | 338.58 | 37.62 | 79 | 21 | 3 | |
| 180 | – | 12 | 427.68 | 35.64 | 81 | 19 | 1 | |
| 181 | – | 11 | 401.28 | 36.48 | 71 | 29 | 2 | |
| 182 | – | 12 | 395.76 | 32.98 | 72 | 28 | 2 | |
| 183 | – | 10 | 372.4 | 37.24 | 71 | 29 | 3 | |
| 184 | – | 13 | 436.8 | 33.6 | 79 | 21 | 3 | |
| 185 | – | 13 | 476.19 | 36.63 | 86 | 14 | 3 | |
| 186 | – | 12 | 439.56 | 36.63 | 75 | 25 | 2 | |
| 187 | – | 10 | 352.8 | 35.28 | 87 | 13 | 3 | |
| 188 | – | 10 | 356.4 | 35.64 | 86 | 14 | 1 | |
| 189 | – | 11 | 390.72 | 35.52 | 82 | 18 | 2 | |
| 190 | – | 9 | 299.25 | 33.25 | 75 | 25 | 3 | |
| 191 | – | 9 | 311.04 | 34.56 | 82 | 18 | 1 | |
| 192 | – | 13 | 441.35 | 33.95 | 79 | 21 | 3 | |
| 193 | – | 12 | 387.6 | 32.3 | 86 | 14 | 2 | |
| 194 | – | 12 | 391.68 | 32.64 | 82 | 18 | 2 | |
| 195 | – | 9 | 302.94 | 33.66 | 73 | 27 | 1 | |
| 196 | – | 9 | 328.32 | 36.48 | 84 | 16 | 3 | |
| 197 | – | 12 | 435.12 | 36.26 | 80 | 20 | 2 | |
| 198 | – | 9 | 326.34 | 36.26 | 84 | 16 | 3 | |
| 199 | – | 9 | 299.88 | 33.32 | 79 | 21 | PUMP GAS | 2 |
| 200 | – | 12 | 399.84 | 33.32 | 87 | 13 | 34.878 | 1 |
Began ethanol fuel testing in higher mixtures
I tried my best to capture as much data as possible to be as scientific as possible. The results do correlate with what I said above however feel free to do your own calculations.  I do a metric crap ton of driving so I am a pretty good measure of a decent commute plus mostly highway driving these results can differ YMMV especially if you drive more city than highway. However my speeds on the highway and my attitude driving in the city vary so perhaps the percentage tracking ive done with the torque app does not always correlate. I tried to be as meticulous as possible when I filled up and tried to make sure I didnt have more than a delta of 2-3 gallons used in difference from tank to tank. It was difficult to do but I wanted solid data. Also please note I got far better at controlling when I filled up over time. The next 200 results will be slightly more consistent on the gallons used. In order to determine the E content of the fuel I used a kit that allows you to do it. They can be found cheap online! (Yes it definitely got old after a while but I kept a good attitude about good data)
I do a metric crap ton of driving so I am a pretty good measure of a decent commute plus mostly highway driving these results can differ YMMV especially if you drive more city than highway. However my speeds on the highway and my attitude driving in the city vary so perhaps the percentage tracking ive done with the torque app does not always correlate. I tried to be as meticulous as possible when I filled up and tried to make sure I didnt have more than a delta of 2-3 gallons used in difference from tank to tank. It was difficult to do but I wanted solid data. Also please note I got far better at controlling when I filled up over time. The next 200 results will be slightly more consistent on the gallons used. In order to determine the E content of the fuel I used a kit that allows you to do it. They can be found cheap online! (Yes it definitely got old after a while but I kept a good attitude about good data)
App used:
Table 2
Ethanol Experimentation Table
| Tank # | E Content | Gallons Used (rounded up) | Distance Traveled | MPG | Highway % | City % | AVG | LTFT (AVG %) | EQV LTFT POST TUNE |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | 12 | 371.28 | 30.94 | 78 | 22 | 12 | (Estimation) | |
| 2 | 29 | 12 | 414.96 | 34.58 | 77 | 23 | 12 | ||
| 3 | 32 | 12 | 386.4 | 32.2 | 75 | 25 | 11 | ||
| 4 | 27 | 10 | 336.7 | 33.67 | 76 | 24 | 12 | ||
| 5 | 31 | 10 | 345.8 | 34.58 | 78 | 22 | 12 | ||
| 6 | 28 | 9 | 292.95 | 32.55 | 80 | 20 | 13 | ||
| 7 | 30 | 12 | 393.12 | 32.76 | 79 | 21 | 13 | ||
| 8 | 27 | 11 | 370.37 | 33.67 | 80 | 20 | 13 | ||
| 9 | 33 | 11 | 346.5 | 31.5 | 73 | 27 | 12 | ||
| 10 | 28 | 10 | 309.4 | 30.94 | 75 | 25 | 13 | ||
| 11 | 30 | 11 | 360.36 | 32.76 | 72 | 28 | 12 | ||
| 12 | 33 | 10 | 316.2 | 31.62 | 76 | 24 | 11 | ||
| 13 | 28 | 12 | 382.2 | 31.85 | 72 | 28 | 12 | ||
| 14 | 30 | 9 | 278.46 | 30.94 | 82 | 18 | 12 | ||
| 15 | 33 | 11 | 388.74 | 35.34 | 72 | 28 | 11 | ||
| 16 | 33 | 11 | 364.32 | 33.12 | 74 | 26 | 12 | ||
| 17 | 28 | 11 | 378.51 | 34.41 | 71 | 29 | 11 | ||
| 18 | 30 | 11 | 356.4 | 32.4 | 81 | 19 | 12 | ||
| 19 | 27 | 9 | 291.6 | 32.4 | 81 | 19 | 11 | ||
| 20 | 33 | 11 | 384.56 | 34.96 | 75 | 25 | 13 | ||
| 21 | 32 | 12 | 371.28 | 30.94 | 75 | 25 | 11 | ||
| 22 | 33 | 10 | 342 | 34.2 | 72 | 28 | 13 | ||
| 23 | 29 | 10 | 349.6 | 34.96 | 82 | 18 | 12 | ||
| 24 | 29 | 11 | 384.56 | 34.96 | 79 | 21 | 12 | ||
| 25 | 27 | 9 | 278.46 | 30.94 | 73 | 27 | 13 | ||
| 26 | 27 | 12 | 393.12 | 32.76 | 77 | 23 | 11 | ||
| 27 | 31 | 12 | 401.76 | 33.48 | 72 | 28 | 11 | ||
| 28 | 28 | 11 | 366.3 | 33.3 | 82 | 18 | 12 | ||
| 29 | 29 | 11 | 384.56 | 34.96 | 72 | 28 | 11 | ||
| 30 | 27 | 11 | 358.05 | 32.55 | 78 | 22 | 12 | ||
| 31 | 32 | 9 | 307.8 | 34.2 | 71 | 29 | 12 | ||
| 32 | 30 | 12 | 414.96 | 34.58 | 79 | 21 | 11 | ||
| 33 | 30 | 11 | 388.74 | 35.34 | 81 | 19 | 13 | ||
| 34 | 33 | 12 | 419.52 | 34.96 | 75 | 25 | 13 | ||
| 35 | 31 | 9 | 281.52 | 31.28 | 81 | 19 | 12 | ||
| 36 | 30 | 9 | 318.06 | 35.34 | 75 | 25 | 12 | ||
| 37 | 33 | 9 | 275.4 | 30.6 | 75 | 25 | 11 | ||
| 38 | 32 | 12 | 408.48 | 34.04 | 81 | 19 | 12 | ||
| 39 | 29 | 9 | 283.5 | 31.5 | 77 | 23 | E30 | 12 | |
| 40 | 33 | 11 | 370.37 | 33.67 | 75 | 25 | 33.14 | 12 | |
| 41 | 43 | 12 | 393.12 | 32.76 | 81 | 19 | 17 | ||
| 42 | 40 | 9 | 269.28 | 29.92 | 76 | 24 | 16 | ||
| 43 | 39 | 9 | 288.36 | 32.04 | 71 | 29 | 17 | ||
| 44 | 38 | 12 | 388.8 | 32.4 | 76 | 24 | 16 | ||
| 45 | 39 | 9 | 300.96 | 33.44 | 81 | 19 | 15 | ||
| 46 | 45 | 12 | 395.16 | 32.93 | 80 | 20 | 16 | ||
| 47 | 46 | 9 | 286.65 | 31.85 | 77 | 23 | 17 | ||
| 48 | 40 | 12 | 369.6 | 30.8 | 72 | 28 | 17 | ||
| 49 | 38 | 12 | 367.2 | 30.6 | 81 | 19 | 17 | ||
| 50 | 44 | 10 | 327.6 | 32.76 | 71 | 29 | 17 | ||
| 51 | 46 | 10 | 311.5 | 31.15 | 82 | 18 | 17 | ||
| 52 | 38 | 12 | 371.28 | 30.94 | 77 | 23 | 15 | ||
| 53 | 37 | 10 | 318.5 | 31.85 | 82 | 18 | 16 | ||
| 54 | 42 | 10 | 302.6 | 30.26 | 71 | 29 | 15 | ||
| 55 | 45 | 11 | 352.44 | 32.04 | 71 | 29 | 15 | ||
| 56 | 42 | 11 | 380.38 | 34.58 | 71 | 29 | 17 | ||
| 57 | 41 | 9 | 294.84 | 32.76 | 76 | 24 | 15 | ||
| 58 | 40 | 10 | 308 | 30.8 | 71 | 29 | 16 | ||
| 59 | 37 | 11 | 342.65 | 31.15 | 82 | 18 | 15 | ||
| 60 | 39 | 9 | 286.65 | 31.85 | 77 | 23 | 17 | ||
| 61 | 41 | 11 | 336.6 | 30.6 | 75 | 25 | 17 | ||
| 62 | 44 | 11 | 372.02 | 33.82 | 76 | 24 | 15 | ||
| 63 | 46 | 11 | 332.86 | 30.26 | 76 | 24 | 17 | ||
| 64 | 43 | 11 | 380.38 | 34.58 | 72 | 28 | 17 | ||
| 65 | 40 | 9 | 280.35 | 31.15 | 73 | 27 | 15 | ||
| 66 | 39 | 12 | 404.04 | 33.67 | 71 | 29 | 17 | ||
| 67 | 46 | 11 | 367.84 | 33.44 | 71 | 29 | 15 | ||
| 68 | 37 | 12 | 393.12 | 32.76 | 81 | 19 | 16 | ||
| 69 | 46 | 12 | 388.8 | 32.4 | 82 | 18 | 15 | ||
| 70 | 40 | 12 | 369.6 | 30.8 | 81 | 19 | 15 | ||
| 71 | 45 | 11 | 352.44 | 32.04 | 79 | 21 | 16 | ||
| 72 | 43 | 10 | 318.5 | 31.85 | 81 | 19 | 17 | ||
| 73 | 43 | 12 | 384.48 | 32.04 | 75 | 25 | 16 | ||
| 74 | 43 | 9 | 272.34 | 30.26 | 82 | 18 | 17 | ||
| 75 | 41 | 12 | 393.12 | 32.76 | 79 | 21 | 17 | ||
| 76 | 43 | 12 | 393.12 | 32.76 | 71 | 29 | 15 | ||
| 77 | 46 | 11 | 332.86 | 30.26 | 74 | 26 | 15 | ||
| 78 | 44 | 9 | 280.35 | 31.15 | 74 | 26 | 15 | ||
| 79 | 45 | 11 | 372.02 | 33.82 | 76 | 24 | 15 | ||
| 80 | 37 | 9 | 303.03 | 33.67 | 72 | 28 | 16 | ||
| 81 | 42 | 12 | 378 | 31.5 | 75 | 25 | 17 | ||
| 82 | 41 | 11 | 329.12 | 29.92 | 78 | 22 | 15 | ||
| 83 | 38 | 11 | 358.16 | 32.56 | 72 | 28 | 16 | ||
| 84 | 37 | 12 | 380.16 | 31.68 | 79 | 21 | 15 | ||
| 85 | 42 | 9 | 280.35 | 31.15 | 72 | 28 | 16 | ||
| 86 | 44 | 9 | 299.7 | 33.3 | 74 | 26 | 15 | ||
| 87 | 46 | 11 | 356.4 | 32.4 | 74 | 26 | 17 | ||
| 88 | 42 | 12 | 401.28 | 33.44 | 76 | 24 | 16 | ||
| 89 | 37 | 12 | 405.84 | 33.82 | 73 | 27 | 15 | ||
| 90 | 42 | 9 | 307.8 | 34.2 | 74 | 26 | 16 | ||
| 91 | 44 | 10 | 315 | 31.5 | 73 | 27 | 17 | ||
| 92 | 41 | 11 | 340.34 | 30.94 | 74 | 26 | 16 | ||
| 93 | 39 | 9 | 304.38 | 33.82 | 76 | 24 | 17 | ||
| 94 | 45 | 11 | 360.36 | 32.76 | 71 | 29 | 17 | ||
| 95 | 45 | 9 | 272.34 | 30.26 | 71 | 29 | 15 | ||
| 96 | 44 | 10 | 336.7 | 33.67 | 78 | 22 | 16 | ||
| 97 | 38 | 10 | 342 | 34.2 | 80 | 20 | 16 | ||
| 98 | 45 | 10 | 325.6 | 32.56 | 73 | 27 | 15 | ||
| 99 | 38 | 11 | 366.3 | 33.3 | 77 | 23 | 17 | ||
| 100 | 37 | 10 | 338.2 | 33.82 | 77 | 23 | 15 | ||
| 101 | 38 | 9 | 277.2 | 30.8 | 77 | 23 | 16 | ||
| 102 | 43 | 11 | 356.4 | 32.4 | 72 | 28 | 15 | ||
| 103 | 43 | 12 | 410.4 | 34.2 | 81 | 19 | 15 | ||
| 104 | 43 | 9 | 296.37 | 32.93 | 75 | 25 | 15 | ||
| 105 | 40 | 10 | 338.2 | 33.82 | 71 | 29 | 17 | ||
| 106 | 45 | 9 | 280.35 | 31.15 | 76 | 24 | 16 | ||
| 107 | 37 | 12 | 373.8 | 31.15 | 72 | 28 | 15 | ||
| 108 | 40 | 12 | 369.6 | 30.8 | 76 | 24 | 16 | ||
| 109 | 42 | 10 | 306 | 30.6 | 78 | 22 | 17 | ||
| 110 | 42 | 10 | 324 | 32.4 | 71 | 29 | 17 | ||
| 111 | 45 | 12 | 390.72 | 32.56 | 73 | 27 | 16 | ||
| 112 | 39 | 9 | 277.2 | 30.8 | 80 | 20 | 17 | ||
| 113 | 39 | 10 | 316.8 | 31.68 | 80 | 20 | 17 | ||
| 114 | 37 | 11 | 348.48 | 31.68 | 76 | 24 | 15 | ||
| 115 | 46 | 11 | 376.2 | 34.2 | 72 | 28 | 16 | ||
| 116 | 40 | 9 | 280.35 | 31.15 | 78 | 22 | 15 | ||
| 117 | 45 | 9 | 293.04 | 32.56 | 82 | 18 | 15 | ||
| 118 | 42 | 12 | 388.8 | 32.4 | 80 | 20 | 15 | ||
| 119 | 44 | 9 | 291.6 | 32.4 | 78 | 22 | 16 | ||
| 120 | 43 | 12 | 390.72 | 32.56 | 82 | 18 | 17 | ||
| 121 | 39 | 9 | 299.7 | 33.3 | 72 | 28 | 17 | ||
| 122 | 43 | 12 | 382.2 | 31.85 | 79 | 21 | 16 | ||
| 123 | 38 | 12 | 378 | 31.5 | 72 | 28 | 17 | ||
| 124 | 39 | 10 | 308 | 30.8 | 78 | 22 | 16 | ||
| 125 | 45 | 10 | 325.6 | 32.56 | 71 | 29 | 16 | ||
| 126 | 38 | 12 | 371.28 | 30.94 | 81 | 19 | 16 | ||
| 127 | 45 | 11 | 358.16 | 32.56 | 80 | 20 | 16 | ||
| 128 | 44 | 11 | 342.65 | 31.15 | 75 | 25 | 16 | ||
| 129 | 44 | 12 | 363.12 | 30.26 | 79 | 21 | 17 | ||
| 130 | 37 | 12 | 399.6 | 33.3 | 76 | 24 | 16 | ||
| 131 | 37 | 10 | 316.8 | 31.68 | 71 | 29 | 17 | ||
| 132 | 42 | 10 | 345.8 | 34.58 | 82 | 18 | 15 | ||
| 133 | 42 | 10 | 325.6 | 32.56 | 82 | 18 | 16 | ||
| 134 | 42 | 12 | 363.12 | 30.26 | 74 | 26 | 17 | ||
| 135 | 41 | 11 | 332.86 | 30.26 | 79 | 21 | 15 | ||
| 136 | 43 | 11 | 338.8 | 30.8 | 73 | 27 | 17 | ||
| 137 | 43 | 10 | 333 | 33.3 | 79 | 21 | 17 | ||
| 138 | 39 | 12 | 401.28 | 33.44 | 81 | 19 | 16 | ||
| 139 | 43 | 10 | 345.8 | 34.58 | 72 | 28 | 15 | ||
| 140 | 44 | 12 | 404.04 | 33.67 | 76 | 24 | 15 | ||
| 141 | 40 | 11 | 360.36 | 32.76 | 82 | 18 | 17 | ||
| 142 | 43 | 9 | 288.36 | 32.04 | 75 | 25 | 17 | ||
| 143 | 39 | 10 | 324 | 32.4 | 74 | 26 | 16 | ||
| 144 | 44 | 12 | 401.28 | 33.44 | 73 | 27 | 17 | ||
| 145 | 42 | 11 | 350.35 | 31.85 | 76 | 24 | 15 | ||
| 146 | 45 | 11 | 367.84 | 33.44 | 78 | 22 | 16 | ||
| 147 | 41 | 12 | 371.28 | 30.94 | 81 | 19 | 16 | ||
| 148 | 43 | 9 | 269.28 | 29.92 | 72 | 28 | 17 | ||
| 149 | 45 | 10 | 306 | 30.6 | 81 | 19 | 15 | ||
| 150 | 45 | 9 | 288.36 | 32.04 | 75 | 25 | 16 | ||
| 151 | 43 | 10 | 320.4 | 32.04 | 71 | 29 | 15 | ||
| 152 | 45 | 11 | 346.5 | 31.5 | 76 | 24 | 15 | ||
| 153 | 38 | 9 | 283.5 | 31.5 | 73 | 27 | 17 | ||
| 154 | 42 | 11 | 360.36 | 32.76 | 73 | 27 | 15 | ||
| 155 | 42 | 9 | 272.34 | 30.26 | 73 | 27 | 16 | ||
| 156 | 39 | 12 | 380.16 | 31.68 | 73 | 27 | 16 | ||
| 157 | 38 | 12 | 395.16 | 32.93 | 78 | 22 | 15 | ||
| 158 | 42 | 11 | 356.4 | 32.4 | 72 | 28 | 17 | ||
| 159 | 41 | 10 | 311.5 | 31.15 | 77 | 23 | E38-E45 | 17 | |
| 160 | 42 | 11 | 352.44 | 32.04 | 72 | 28 | 32.12 | 16 | Tune occurred here so LTFT Changed |
| 161 | 81 | 11 | 276.76 | 25.16 | 77 | 23 | FINALLY | 5 | 27 |
| 162 | 75 | 9 | 263.07 | 29.23 | 73 | 27 | TUNED | 4 | 27 |
| 163 | 83 | 11 | 314.16 | 28.56 | 72 | 28 | FOR | 5 | 26 |
| 164 | 70 | 11 | 345.95 | 31.45 | 81 | 19 | E85 | 3 | 27 |
| 165 | 81 | 12 | 372.96 | 31.08 | 80 | 20 | 3 | 27 | |
| 166 | 71 | 11 | 301.18 | 27.38 | 78 | 22 | 4 | 25 | |
| 167 | 78 | 9 | 259.74 | 28.86 | 72 | 28 | 5 | 23 | |
| 168 | 76 | 11 | 288.75 | 26.25 | 72 | 28 | 4 | 23 | |
| 169 | 83 | 11 | 277.2 | 25.2 | 74 | 26 | 3 | 26 | |
| 170 | 79 | 9 | 275.4 | 30.6 | 80 | 20 | 3 | 26 | |
| 171 | 72 | 10 | 277.5 | 27.75 | 73 | 27 | 5 | 25 | |
| 172 | 73 | 10 | 282.2 | 28.22 | 76 | 24 | 4 | 27 | |
| 173 | 69 | 12 | 369.36 | 30.78 | 73 | 27 | 3 | 26 | |
| 174 | 76 | 10 | 277.5 | 27.75 | 72 | 28 | 3 | 27 | |
| 175 | 77 | 9 | 272.16 | 30.24 | 76 | 24 | 3 | 23 | |
| 176 | 78 | 11 | 324.72 | 29.52 | 74 | 26 | 3 | 23 | |
| 177 | 81 | 9 | 249.48 | 27.72 | 82 | 18 | 5 | 25 | |
| 178 | 81 | 12 | 341.88 | 28.49 | 78 | 22 | 4 | 26 | |
| 179 | 78 | 12 | 323.4 | 26.95 | 78 | 22 | 3 | 27 | |
| 180 | 69 | 10 | 294 | 29.4 | 80 | 20 | 4 | 25 | |
| 181 | 79 | 10 | 287 | 28.7 | 79 | 21 | 5 | 24 | |
| 182 | 81 | 12 | 346.56 | 28.88 | 80 | 20 | 5 | 26 | |
| 183 | 80 | 9 | 259.92 | 28.88 | 75 | 25 | 4 | 25 | |
| 184 | 73 | 10 | 259 | 25.9 | 78 | 22 | 3 | 25 | |
| 185 | 72 | 9 | 259.74 | 28.86 | 72 | 28 | 3 | 23 | |
| 186 | 83 | 12 | 369.36 | 30.78 | 73 | 27 | 3 | 23 | |
| 187 | 72 | 11 | 308.88 | 28.08 | 78 | 22 | 5 | 26 | |
| 188 | 71 | 10 | 298.8 | 29.88 | 78 | 22 | 4 | 26 | |
| 189 | 79 | 9 | 263.07 | 29.23 | 71 | 29 | 4 | 23 | |
| 190 | 75 | 12 | 333 | 27.75 | 80 | 20 | 5 | 27 | |
| 191 | 83 | 9 | 269.73 | 29.97 | 80 | 20 | 4 | 23 | |
| 192 | 77 | 11 | 285.12 | 25.92 | 78 | 22 | 5 | 23 | |
| 193 | 81 | 9 | 243 | 27 | 77 | 23 | 5 | 25 | |
| 194 | 72 | 10 | 273 | 27.3 | 74 | 26 | 5 | 23 | |
| 195 | 82 | 12 | 301.92 | 25.16 | 72 | 28 | 4 | 25 | |
| 196 | 75 | 11 | 332.64 | 30.24 | 78 | 22 | 3 | 24 | |
| 197 | 79 | 11 | 324.72 | 29.52 | 74 | 26 | 5 | 24 | |
| 198 | 74 | 12 | 337.44 | 28.12 | 71 | 29 | 3 | 27 | |
| 199 | 75 | 12 | 318.24 | 26.52 | 78 | 22 | E85 | 4 | 26 |
| 200 | 83 | 10 | 283.5 | 28.35 | 78 | 22 | 28.48 | 3 | 25 |
So conclusions from the test say that no ethanol has not harmed my vehicle its still purring like a kitten and still running on it and have been running on it since the mileage indicated prior to now 398,776 miles  No major breakdowns. Maybe ill get a boroscope and take pics of the cylinders one day
No major breakdowns. Maybe ill get a boroscope and take pics of the cylinders one day  Last time I removed the head and valve cover for kicks and giggles and because I wanted to prematurely replace the gaskets of both. The cylinders were extremely clean to my surprise. Ethanol is very clean burning. Project farm has done some interesting testing too and one with a see through engine… Worth the watch!
Last time I removed the head and valve cover for kicks and giggles and because I wanted to prematurely replace the gaskets of both. The cylinders were extremely clean to my surprise. Ethanol is very clean burning. Project farm has done some interesting testing too and one with a see through engine… Worth the watch!
Reason for damage seen in video. Ethanol absolutely eats felt and cellulosic based gaskets such as card board based gaskets. Be mindful of this issue
I actually got my lawn mower to run on it by drilling the jets out and now it runs E85. Somethings ive noticed running it in cars and lawn mowers is the smell of hand sanitizer when the exhaust is a bit rich… Makes me feel good and not like im about to keel over and die.
Production is the problem
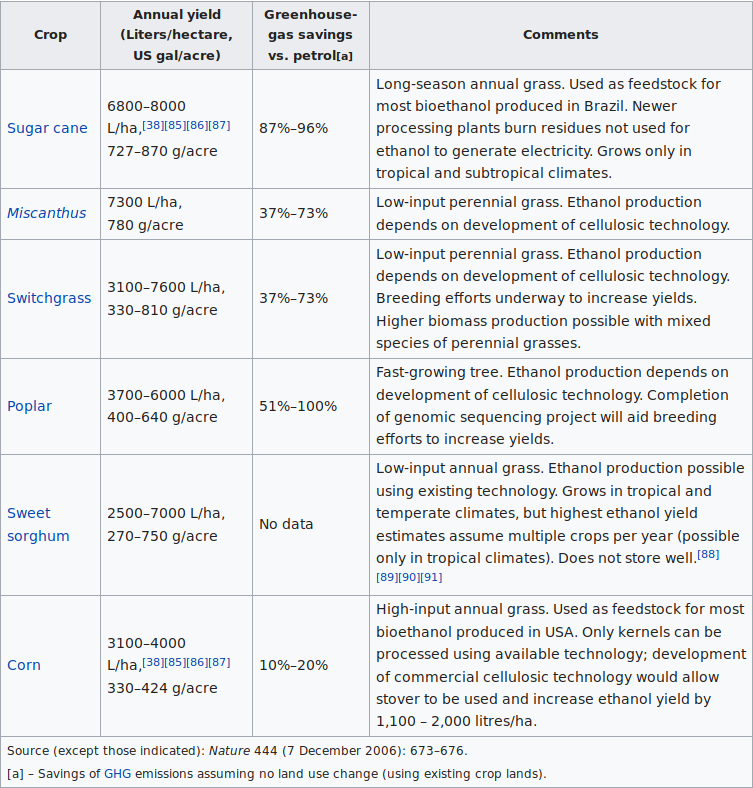
Current yields are around 41-44 percent. The problem with this is if we compare this to in terms of GPE or Gasoline Production Equivalent its closer 27 to 34 percent from source to product using the corn to ethanol methods.
This can be changed here is a charge from wikipedia thats very accurate.
If you do the math you see that the methods that are worth it are poplar and above. There is a ton of research into creating cellulose ethanol based production where we ferment directly from the plant matter instead of first having to extract the sugar which is only really effective with sugar beets and sugar cane. The grasses can grow in the desert regions of the west without man made irrigation. This is why its exciting research and I hope to see it develop. This might be a great fuel to reduce our overall lifecycle emissions and since its so clean burning it will likely improve car longevity and possibly alleviate the carbon issue on intake valves with direction injection.
There are other alcohols.
In the future I may right about them as being possible biofuels.
Pentanol: A five carbon alcohol part of the OH Functional sub group of amyl alcohols. It has an energy density between gasoline and diesel and would improve MPG if used. It also hase a consistency closer to that of kerosene due to being a fusel oil. However it can be spark ignited and has a decent AKI.
Butanol: A four carbon alcohol of the OH functional sub group Butyl contains roughly 1-3 percent less energy than gasoline.
Propanol: A 3 carbon alcohol with a energy content between ethanol and butanol. Also very clean burning.
All of which can be fermented from cellulose and can also by means of a catalyst be converted into from ethanol. They are equally worth their weight in gold in terms of research.
Potentional bio or synthetic gasolines
Possibly good fuels. Not enough research data to be conclusive
Cleaner Gasolines
https://en.wikipedia.org/wiki/Biogasoline
https://en.wikipedia.org/wiki/Synthetic_fuel
Final message
This post is probably not as grand as I dreamed. My time is limited but I wanted to share some knowledge. Ive decided to leave the rest of the data to be exposed by questions and I will gladly answer them. SO even if your not a gear head or you are I hope you learned something about ethanol today. If anything you can stop spreading misinformation. This fuel is a fantastic fuel and is worth its extra cost to develop. After all its not like gasoline wasnt equally expensive to develop up to the point its at now. Heck we had what 80 years or more to get that right and weve barely been thoroughly researching ethanol since 25-30 years ago. We can figure it out! The earth matters not just for us but for our children. Using our resources to figure out how to improve our impact should never be a bad thing but it also shouldnt come at an expense of the american people like Emissions Testing and Diesel emissions regulations have become for people not only in costly repairs but forced obsolescence. More on this in another post. Let me know if you liked this and as always im open to questions below! I know this post will need some cleaning up. I just rather not keep it in my inbox in drafting. People can know now 
POST TWO TOPIC – Biodiesel
A great fuel made from used oils and waste products
Other Resources (Growing)
List
https://www.greencarcongress.com/2008/01/study-compares.html
https://www.corvetteonline.com/news/the-benefits-of-e85-as-a-power-adder/
https://www.greencarcongress.com/2019/06/20190601-e15.html
Various Wikipedia sources used and read during the long investigation:
“Towards Sustainable Production and Use of Resources: Assessing Bio fuels” (PDF). United Nations Environment Programme. 16 October 2009. Archived from the original (PDF) on 22 November 2009. Retrieved 24 October 2009.
Renewable Fuels Association (6 March 2012). “Acelerating Industry Innovation – 2012 Ethanol Industry Outlook” (PDF).
Renewable Fuels Association. Archived from the original (PDF) on 14 May 2012. Retrieved 18 March 2012. See pp. 3, 8, 10 22 and 23 .
AMIS Market Monitor No. 48 – May 2017, http://www.amis-outlook.org/fileadmin/user_upload/amis/docs/Market_monitor/AMIS_Market_Monitor_Issue_47.pdf
“Gasoline Gallon Equivalent (GGE) Definition”. energy.gov. Retrieved 12 October 2011.
“Alternative Fuels Data Center – Fuel Properties Comparison” (PDF). Alternative Fuels Data Center . 29 October 2014.
“The Renewable Path to Energy Security” (PDF). Images1.americanprogress.org. Retrieved 20 January 2015.
“Deforestation diesel – the madness of biofuel” (PDF). Retrieved 27 August 2011.
Youngquist, W. Geodestinies , National Book Company, Portland, Oregon, p.499
“The dirty truth about biofuels”. Oilcrash.com. 14 March 2005. Retrieved 27 August 2011.
Kinver, Mark (18 September 2006). “Biofuels look to the next generation”. BBC News . Retrieved 27 August 2011.
O. R. Inderwildi; D. A. King (2009). “Quo Vadis Biofuels”. Energy & Environmental Science . 2 (4): 343.
“Industrial & Environmental” (PDF). Bio.org. Archived from the original (PDF) on 12 February 2006. Retrieved 20 January 2015.
“World Energy Outlook 2006” (PDF). Worldenergyoutlook.org. Archived from the original (PDF) on 28 September 2007. Retrieved 20 January 2015.
“World Fuel Ethanol Analysis and Outlook” (PDF). Meti.go.jp. Archived from the original (PDF) on 28 March 2016. Retrieved 20 January 2015.
“(grainscouncil.com, Biofuels_study 268 kB pdf, footnote, p 6)” (PDF). 18 July 2008. Archived from the original (PDF) on 18 July 2008. Retrieved 27 August 2011.
[1] Archived 9 May 2008 at the Wayback Machine
Martin LaMonica (12 June 2008). “Algae farm in Mexico to produce ethanol in '09”. News.cnet.com. Retrieved 27 August 2011.
“New Enzyme for More Efficient Corn Ethanol Production”. Green Car Congress. 30 June 2005. Retrieved 14 January 2008.
“Ethanol”. University of Illinois Extension . Retrieved 10 July 2017.
Volpato Filho, Orlando (September 2008). Gasoline C made with Hydrous Ethanol . XVI SIMEA 2008 - Simpósio Internacional de Engenharia Automotiva. Sao Paolo. Retrieved 10 July 2017.
“Modern Corn Ethanol plant description” (PDF).
Stacey, Neil T.; Hadjitheodorou, Aristoklis; Glasser, David (19 September 2016). “Gasoline Preblending for Energy-Efficient Bioethanol Recovery”. Energy & Fuels . 30 (10): 8286–8291.
doi:10.1021/acs.energyfuels.6b01591. ISSN 0887-0624.
W. Horn and F. Krupp. Earth: The Sequel: The Race to Reinvent Energy and Stop Global Warming. 2006, 85
This is shown for 25 °C (77 °F) in a gasoline-ethanol-water phase diagram, Fig 13 of Päivi Aakko; Nils-Olof Nylund. “Technical View on Biofuels for Transportation – Focus on Ethanol End-Use Aspects” (PDF). Archived from the original (PDF) on 3 December 2007. Retrieved 14 January 2008.
“Water Phase Separation in Oxygenated Gasoline” (PDF). Epa.gov. Archived from the original (PDF) on 9 February 2015. Retrieved 20 January 2015.
“Home Mini-Refinery Makes Ethanol & Biodiesel Simultaneously”. Gas2.0. 4 November 2008. Retrieved 4 November 2008.
“Micro Fueler Is First Ethanol Kit for Brewing Backyard Biofuels on the Cheap”. PopularMechanics. 8 May 2008. Archived from the original on 9 May 2008. Retrieved 8 May 2008.
“Alternative Fuels Data Center: Ethanol”. Afdc.energy.gov. Retrieved 20 January 2015.
“U.S. Energy Information Administration (EIA)”. Archived from the original (PDF) on 21 August 2008. Retrieved 2016-02-09.
“Ethanol in Petrol”. Royal Automobile Association of South Australia. February 2004. Archived from the original on 9 June 2007. Retrieved 29 April 2007.
“EPA Info”. US EPA. 7 March 2011. Archived from the original on 25 June 2009. Retrieved 27 August 2011.
J. Goettemoeller; A. Goettemoeller (2007). Sustainable Ethanol: Biofuels, Biorefineries, Cellulosic Biomass, Flex-Fuel Vehicles, and Sustainable Farming for Energy Independence . Prairie Oak Publishing, Maryville, Missouri. p. 42. ISBN 978-0-9786293-0-4.
“EPA Mileage”. Fueleconomy.gov. Retrieved 27 August 2011.
“Changes in Gasoline IV, sponsored by Renewable Fuels Foundation” (PDF). Archived from the original (PDF) on 2 August 2012. Retrieved 27 August 2011.
Roman M. Balabin; et al. (2007). “Molar enthalpy of vaporization of ethanol–gasoline mixtures and their colloid state”. Fuel . 86 (3): 323. doi:10.1016/j.fuel.2006.08.008.
“Sustainable biofuels: prospects and challenges”. The Royal Society. January 2008. Archived from the original (PDF) on 5 October 2008. Retrieved 27 September 2008. Policy document 01/08. See 4.3.1 Vapour pressure and bioethanol and Figure 4.3 for the relation between ethanol content and vapor pressure.
Ethanol Promotion; Information Council (27 February 2007). “When is E85 not 85 percent ethanol? When it’s E70 with an E85 sticker on it”. AutoblogGreen. Retrieved 24 August 2008.
“Ethanol fuel and cars”. Interesting Energy Facts. 23 September 2008. Retrieved 23 September 2008.
Vägverket (Swedish Road Administration) (30 May 2007).
“Swedish comments on Euro 5/6 comitology version 4, 30 May 2007: Cold Temperature Tests For Flex Fuel Vehicles” (PDF). ec.europa.eu . European Commission. Archived from the original (PDF) on 3 October 2008. Retrieved 23 September 2008.
“Here comes the ‘Flex’ vehicles third generation” (PDF). Revista Brasileira de BioEnergia (in Portuguese and English). August 2008. Archived from the original (PDF) on 3 October 2008. Retrieved 23 September 2008. Ano 2, No. 3 (every article is presented in both English and Portuguese)
Agência Estado (10 June 2008).
doi:
10.11606/D.86.2007.tde-07052008-115336. Retrieved 5 October 2008. PhD Dissertation Thesis, pp. 81–82
“2011 Ethanol Industry Outlook: Building Bridges to a More Sustainable Future” (PDF). Renewable Fuels Association. 2011. Archived from the original (PDF) on 28 September 2011. Retrieved 30 April 2011. See pages 2–3, 10–11, 19–20, and 26–27 .
Matthew L. Wald (13 October 2010).
“A Bit More Ethanol in the Gas Tank”.
The New York Times* . Retrieved 14 October 2010.
Fred Meier (13 October 2010).
“EPA allows 15% ethanol in gasoline, but only for late-model cars”. USA Today . Retrieved 14 October 2010.
[2] Scania PRESSInfo, 21 May 2007
Archived 20 March 2009 at the
“Ethanol Producer Magazine – The Latest News and Data About Ethanol Production”. Ethanolproducer.com. Retrieved 20 January 2015.
Cohn, D.R.; Bromberg, L.; Heywood, J.B. (20 April 2005),
“Direct Injection Ethanol Boosted Gasoline Engines: Biofuel Leveraging for Cost Effective Reduction of Oil Dependence and CO2 Emissions. MIT Report PSFC/JA-06-16” (PDF), MIT Energy Initiative , archived from the original (PDF) on 2 June 2013, retrieved 23 November 2014 Stokes, J.; Lake, T. H.; Osborne, R. J. (16 October 2000).
“A Gasoline Engine Concept for Improved Fuel Economy -The Lean Boost System”. SAE Paper 2001-01-2901 . SAE Technical Paper Series. 1 . Sae.org.
doi:10.4271/2000-01-2902. Retrieved 27 August 2011.
M. Brusstar; M. Bakenhus.
“Economical, High-Efficiency Engine Technologies for Alcohol Fuels” (PDF). U.S. Environmental Protection Agency. Retrieved 14 January 2008.
Voelcker, John (14 June 2016).
“Nissan takes a different approach to fuel cells: ethanol”. Green Car Reports . Retrieved 16 June 2016.
F.O. Lichts.
“Industry Statistics: 2010 World Fuel Ethanol Production”. Renewable Fuels Association. Retrieved 30 April 2011.
“2009 Global Ethanol Production (Million Gallons)” (PDF). F.O. Licht, cited in Renewable Fuels Association, Ethanol Industry Overlook 2010, pp. 2 and 22. 2010. Archived from the original (PDF) on 18 July 2011. Retrieved 12 February 2011.
F.O. Licht. “2007 and 2008 World Fuel Ethanol Production”. Renewable Fuels Association. Archived from the original on 8 April 2008. Retrieved 17 April 2010.
Joel K. Bourne, Jr. “Biofuels”. Ngm.nationalgeographic.vom. Retrieved 20 January 2015.
[3] Archived 8 September 2015 at the Wayback Machine
“01.26.2006 - Ethanol can replace gasoline with significant energy savings, comparable impact on greenhouse gases”. Berkeley.edu. Retrieved 20 January 2015.
“oregon.gov, biomass forum”. Oregon.gov. 27 March 2009. Archived from the original on 28 August 2011. Retrieved 27 August 2011.
M. Wang; C. Saricks; D. Santini. “Effects of Fuel Ethanol Use on Fuel-Cycle Energy and Greenhouse Gas Emissions” (PDF). Argonne National Laboratory. Retrieved 7 July 2009.
M. Wang. “Energy and Greenhouse Gas Emissions Effects of Fuel Ethanol” (PDF). Retrieved 7 July 2009.
Davidson, Keay (18 April 2007). “Study warns of health risk from ethanol”. San Francisco Chronicle . Retrieved 7 July 2009.
“Clearing the air on ethanol”. Environmental Science & Technology. 18 April 2007. Archived from the original on 27 October 2008. Retrieved 14 January 2008.
M. Z. Jacobson (14 March 2007). “Effects of Ethanol (E85) vs. Gasoline Vehicles on Cancer and Mortality in the United States”. ACS Publications. Retrieved 14 January 2008.
Nguyen, H. (2001). “Atmospheric alcohols and aldehydes concentrations measured in Osaka, Japan and in Sao Paulo, Brazil”. Atmospheric Environment . 35 (18): 3075–3083.
doi:10.1016/S1352-2310(01)00136-4.
“Part One” (PDF). Archived from the original (PDF) on 24 November 2016. Retrieved 27 August 2011.
“Bioethanol Production and Use Creating Markets for Renewable Energy Technologies” (PDF). eubia.org . EU, RES Technology Marketing Campaign, European Biomass Industry Association EUBIA. 2007. Archived from the original (PDF) on 28 November 2007.
“Biofuels Deemed a Greenhouse Threat”. The New York Times . Retrieved 20 January 2015.
Joseph Fargione (29 February 2008). “Land Clearing and the Biofuel Carbon Debt”. Science . 319 (5867): 1235–1238. doi:10.1126/science.1152747. PMID 18258862.
D. Morrison (18 September 2006). “Ethanol fuel presents a corn-undrum”. University of Minnesota. Archived from the original on 22 September 2007. Retrieved 14 January 2008.
“Lula calls for ethanol investment”. BBC. 4 June 2007. Retrieved 14 January 2008.
“Sweet sorghum: A Water Saving BioEnergy Crop” (PDF). International Crops Research Institute for the SemiArid Tropics. Retrieved 14 January 2008.
“RP INVESTOR TO PUT UP PIONEERING SWEET SORGHUM ETHANOL PLANT”. Manila Bulletin. 25 October 2006. Archived from the original on 12 February 2008. Retrieved 14 January 2008.
G. C. Rains; J. S. Cundiff; G. E. Welbaum (12 September 1997).
“Sweet Sorghum for a Piedmont Ethanol Industry”.
“ICRISAT develops sweet sorghum for ethanol production”. 12 August 2004. Archived from the original on 15 December 2007.
“Energy Security” (PDF). Ethanol.org. Archived from the original (PDF) on 23 April 2012.
M. Turon (25 November 1998). Ethanol as Fuel: An Environmental and Economic Analysis . U.C. Berkeley, Chemical Engineering.
“Ethanol Can Contribute to Energy and Environmental Goals” (PDF). Ethanol.org. Archived from the original (PDF) on 23 April 2012.
“Energy INFOcard”. Eia.doe.gov. Retrieved 27 August 2011.
“Ethanol Lowers Gas Prices 29–40 Cents Per Gallon”. Renewableenergyworld.com.
“ALMS Corvettes going green with E85 fuel in 2008 - USATODAY.com”. Usatoday30.usatoday.com.
Fox Sports. “NASCAR”. FOX Sports . Retrieved 20 January 2015.[ permanent dead link ]
“Impact of Improved Stoves and Fuels on IAP”, CEIHD Center for Entrepreneurship in International Health and Development.
Jim Lane (1 August 2013). “INEOS Bio produces cellulosic ethanol from waste, at commercial scale – print-friendly”. Biofuels Digest. Retrieved 15 June 2014.
“Ethanol production using genetically engineered bacterium”. Azom.com. 23 September 2010. Retrieved 23 April 2012.
“Air Pollution Rules Relaxed for U.S. Ethanol Producers”. Environmental News Service. 12 April 2007. Retrieved 26 June 2009.
“Nano-spike catalysts convert carbon dioxide directly into ethanol | ORNL”. www.ornl.gov . Retrieved 11 November 2016.
Reputable Studies Section
Sources
https://www.sciencedirect.com/science/article/abs/pii/S0167779907000492
https://www.sciencedirect.com/science/article/pii/S0960852409015119
https://www.osti.gov/biblio/1218382
https://www.pnas.org/content/105/2/464.short
http://www.ijabe.org/index.php/ijabe/article/view/168
https://onlinelibrary.wiley.com/doi/abs/10.1002/biot.200600067
https://www.sciencedirect.com/science/article/pii/S0961953407000396
https://link.springer.com/chapter/10.1007/978-1-4419-7145-6_13
https://www.sciencedirect.com/science/article/abs/pii/S036054421300683X
https://www.sciencedirect.com/science/article/abs/pii/S016777990600254X
https://www.nature.com/articles/nrg2336
https://www.pnas.org/content/106/5/1368.short
https://www.osti.gov/biblio/6396491
https://science.sciencemag.org/content/251/4999/1318
https://science.sciencemag.org/content/311/5760/506
https://onlinelibrary.wiley.com/doi/abs/10.1002/bbb.269
https://www.nature.com/articles/nbt0108-8
https://www.nature.com/news/cellulosic-ethanol-fights-for-life-1.14856
https://www.sciencedirect.com/science/article/pii/S0960852407007730
 today is not the day . Also Let me know if you guys want this section to turn into a how to distill ethanol aka how to distill moonshine section. I do not endorse nor encourage tax evasive behavior. If you decide to distill ethanol you accept the liabilities of your own decision making
today is not the day . Also Let me know if you guys want this section to turn into a how to distill ethanol aka how to distill moonshine section. I do not endorse nor encourage tax evasive behavior. If you decide to distill ethanol you accept the liabilities of your own decision making